Hawkins is routinely appointed to investigate the cause of fires occurring at farms. This particular case study discusses some unusual aspects that caused an exothermic reaction, and relates to the use of calcium oxide (also known as quicklime), as an animal-friendly sanitization product. Calcium oxide is most often used in the form of a white powder, made from the thermal decomposition of materials like limestone and seashells. It can be used to disinfect and sanitize, because it creates a dry environment where bacteria will not thrive. It is also often used in agricultural settings as a fertilizer and soil conditioner. Quicklime has previously been used as a weapon of war, it is thought to have been one of the components of Greek Fire. During the reign of Henry III, the English Navy destroyed an invading French fleet by blinding them with clouds of quicklime. In fiction, Bernard Cornwell’s 19th Century hero, Richard Sharpe used quicklime that he made by burning oyster shells, to stave off the French army (Sharpe’s Siege).
BACKGROUND INFORMATION AND DISCOVERY OF THE FIRE
Before Hawkins carried out an examination of the building, the circumstances of the incident were discussed with the two workers who discovered the fire:
The fire occurred in a wooden building, which housed laying hens, and had an open-air veranda on one side.
At approximately 14:30, the two workers cleared the birds from the veranda, and then spread a bag of calcium oxide over the floor of the veranda to disinfect it. They then put fresh straw over the floor before letting the birds back onto the veranda. Finally, the workers left the building to carry out work elsewhere.
At 17:00, one of the workers noticed smoke coming from the veranda of the building. He went to investigate and saw smoke and flames at the north-western corner of the veranda.
Though there were electric lights in the veranda, these were switched off at the time of the fire. The workers said there had neither been recent work on, nor problems with, the fixed wiring in the building.
One of the workers was a smoker, but he stated that he did not smoke inside the buildings.
INVESTIGATION
Damage attributable to direct fire attack was limited to the veranda.

I noted several piles (approximately 20 cm in diameter) of a white powder amongst the straw in the veranda. I did not find any smoker’s materials in the area of fire damage, nor did I find any around the other farm buildings.

Quicklime is not typically considered a fire hazard, but calcium oxide will react violently with moisture and give off heat (an exothermic reaction). It was initially unclear, however, whether sufficient heat could be generated by such an exothermic reaction to ignite the straw.
I conducted tests to assess if the exothermic reaction of calcium oxide and water could ignite straw. A layer of clean, dry straw was placed on a metal tray. A pile of 500g of calcium oxide was placed on the straw, and then 500ml of water was added. A layer of straw was placed on top of this; there were four temperature sensors (Channels 1 – 4) inside the top layer of straw.
Figure 1 shows the temperatures recorded during this test. The temperature of Channel 1, Channel 3 and Channel 4 increased rapidly once water was added to the calcium oxide. Channel 3 and Channel 4 reached a maximum of approximately 100°C and then plateaued, while Channel 1 continued to rise until it reached approximately 370°C, about nine minutes after water was added. Initially steam came from the straw, but this soon subsided.
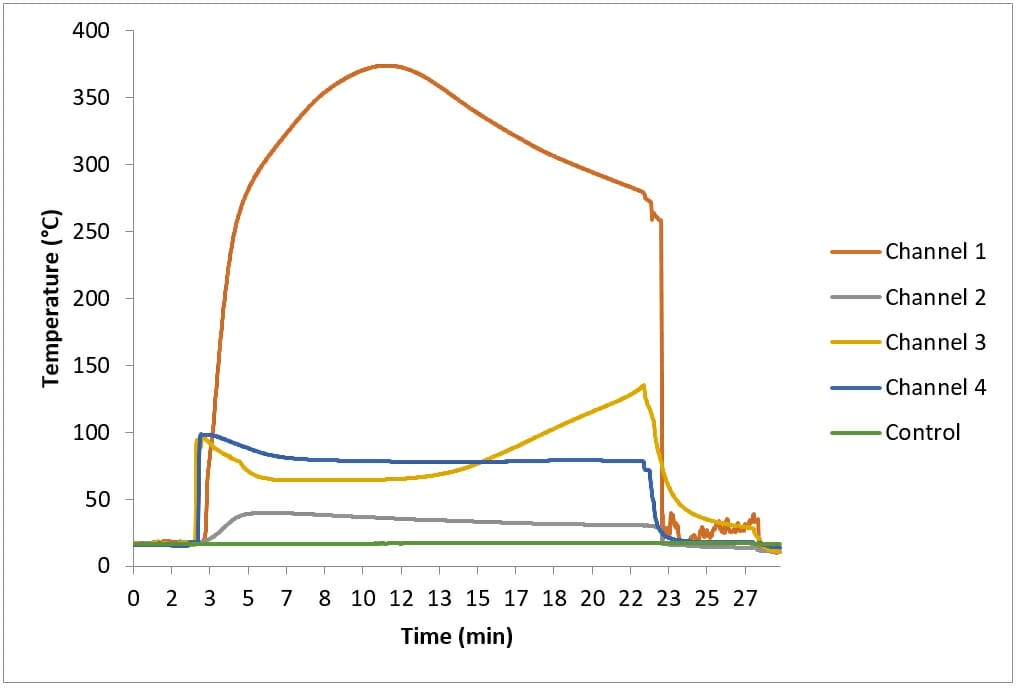
Approximately eighteen minutes after the water was added “crackling” was heard coming from the straw, and smoke began to appear. I removed the top layer of straw and saw that the straw underneath it was blackened. However, moving the top layer of straw allowed air to reach the blackened straw, and caused it to glow. A flaming fire quickly developed.


CONCLUSIONS
The tests showed that calcium oxide, when mixed with water, reacts exothermically, and represents a viable ignition source for the straw bedding material on the veranda.
When I reviewed the instructions provided with the calcium oxide, I noticed that they failed to specifically mention the risk of fire. However, the instructions did stipulate that the product must be spread evenly, and that the ground should be wetted prior to treatment.
If the calcium oxide had been spread evenly onto a pre-wetted surface, the exothermic reaction would have occurred rapidly, and heat would have been lost to the surroundings. The farm workers’ failure to spread the calcium oxide in a way that would ensure a rapid reaction was then compounded by the placement of straw on top of the calcium oxide. This reduced the amount of heat lost to the surroundings, and instead provided a readily ignitable material.
Hawkins has prepared a letter for the manufacturers, which detailed these findings and suggested that manufacturers of quick lime review the advice provided with the product–highlighting the need to spread the calcium oxide evenly to reduce the risk of fire.
ABOUT THE AUTHOR
Dr Ian Tatner is a Chartered Engineer (CEng) with a PhD in Corrosion Fatigue of Stainless Steel. Though his career began in the offshore oil and gas pipeline installation industry, Ian joined Hawkins in early 2009, and has been investigating fires and explosions, as well as pedestrian falls, ever since. He has seen a broad range of cases from fires in domestic properties, to more complex vehicle fires and factory explosions. Ian has also investigated numerous pedestrian falls in both public and private buildings.




