Hawkins has investigated numerous fires where the most probable cause has been the self-heating of rags, cloths or other similar materials that were impregnated with drying oils, such as linseed oil.
Photographs above show the seat of fire in an entrance hallway where pieces of burnt material were found on the doormat, left by a contractor who had been applying a drying oil to the wooden flooring.
DRYING OILS
Drying oils are commonly used in the treatment of wooden flooring. The containers in which the oils are supplied usually indicate that the contents pose a risk of self-ignition. The recommended disposal for sanding dust or oil-wetted cloths is for them to be incinerated or soaked in water and disposed of in tightly closed metal containers after use. In reality however, used cloths are often left in piles or loosely packed into bags by contractors or those carrying out home improvements upon completion of work.
THE SELF-HEATING PROCESS
The spontaneous combustion – or self-heating – of fats and oils that can readily oxidise in an exothermic reaction is a well-known cause of fires. Drying oils harden to a tough, solid film after a period of exposure to air. The self-heating propensity of a substance is generally proportional to the degree of unsaturation of the oil molecules, since this increases the ease of oxidation. When an exothermic reaction occurs in a mass of materials, the temperature will rise, provided that the rate at which heat is produced in the mass exceeds the rate at which heat is lost.
The rate of heat loss is proportional to the surface area of the mass, whereas the amount of heat in a chemical reaction is proportional to the volume (assuming that there are sufficient reactants present). The rate at which a chemical reaction proceeds is also governed by temperature. Generally, the rate of reaction doubles for every 10°C increase in temperature.
These characteristics mean that piles of materials undergoing an exothermic reaction are susceptible to self-heating. This is because it is difficult for the material at the centre of the pile to lose heat to its surroundings, causing the temperature to rise. This, in turn, increases the rate of reaction and heat production and can ultimately bring the material to its auto ignition temperature, at which point a fire will occur without the need for an external ignition source such as a flame.
For self-heating to occur due to oxidation, the mass of material requires ventilation to provide the required oxygen. This is not possible, for instance, with a container of liquid linseed oil, since oxygen is only available at the surface. A rag covered with a linseed type oil, however, very significantly increases the surface area where there is contact between oxygen and the oil. While it may be very difficult to quantify the exposed surface area of an oil-covered rag, it is clear that the surface area to volume ratio is much greater than that for liquid oil in a container. The effect of types of fibres onto which such oils are impregnated may also be expected to have some effect on self-heating potential.
EXPERIMENTAL EVIDENCE OF SELF-HEATING
Self-heating experiments have been carried out by Hawkins. Pieces of material from a stockinette roll of cloth were used and wetted with a WOCA Colour Oil (containing drying oils) in ratios from 1:1 to 1:1.4 (mass of cloth:mass of oil). The average weight of the stockinette samples used for each experiment was 103 g, which consisted of three 400 mm long pieces of stockinette and one piece 200 mm in length.

Once the cloths had been wetted with the calculated weight of colour oil and the oil absorbed into the stockinette, the cloths were placed into small piles, such as might be left behind after finishing oiling work. Each pile was instrumented with two thermocouples; the first placed at the junction between the mat and the cloth and the second placed in the centre of the pile. The piles were left in ambient laboratory conditions for a period of up to 66 hours.
The temperature profiles over the period of the experiment were measured. No reaction or heating of the cloth and oil occurred within the first 45 hours of the experiment. Self-heating within the cloths started at around 46 hours for each pile. Once all of the self-heating reactions had taken place, the piles of cloth were checked for signs of change or smouldering ignition. There was discolouration within the cloths where the self-heating had occurred.
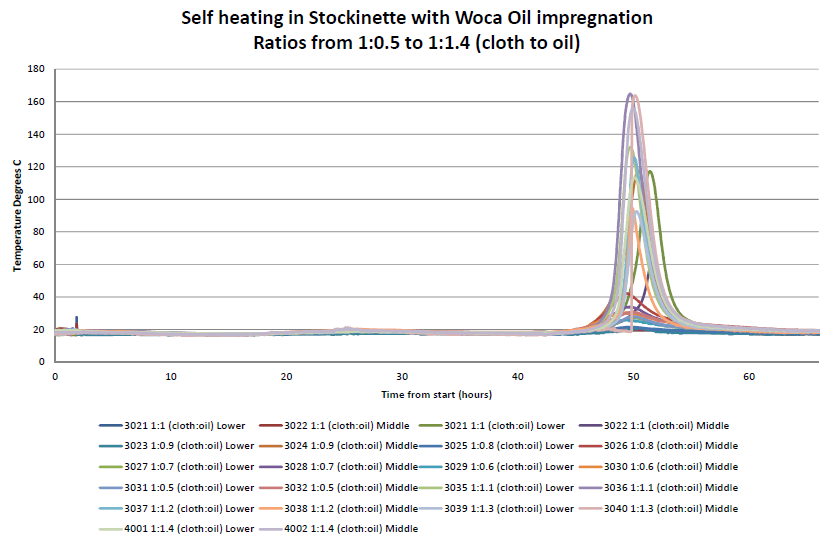
In summary, an exothermic self-heating reaction was observed in all cloth samples to a certain degree under the conditions of the experiment. The self-heating within the piles ceased after 54 hours with peak temperature being achieved between 49 and 51 hours. The reaction was such that a temperature of 140 °C above ambient temperature was observed. The piles were inspected after each experiment and they showed signs of discolouration where self-heating had occurred. Whilst none of the piles of cloth ignited, if the piles were larger and/or the ambient temperature higher, then self-heating leading to combustion might have occurred.

The tests were carried out on relatively small piles of cloths and therefore will not have represented the range of real-life scenarios whereby pile size, material type, arrangement and distribution of oil will vary and other substances may be present on the material. With regards to the latter, previous tests carried out by Hawkins found that the time taken for self-heating to occur for rags soaked in a similar WOCA branded Colour Oil decreased if the rags were also contaminated with white spirit. Self-heating occurred up to 58 hours for rags soaked in Colour Oil alone, but those soaked in Colour Oil and white spirit self-heated after approximately 6 hours, with two of those tests resulting in ignition.
INVESTIGATING IGNITION DUE TO SELF-HEATING
Any cloths or rags left in a pile or in a bin or bag have the propensity to self-heat and pose a risk of fire. The fire investigator should consider it plausible that rags left wetted with drying oil- from anywhere from 1 hour up to even 2 or 3 days could be a potential source of ignition.
ABOUT THE AUTHOR
Since joining Hawkins in 2006, Dr Claire Mann has specialised in the investigation of causes of fires and explosions. These cases have varied greatly in scale from relatively small domestic incidents to large commercial fires. Her background in chemistry makes her ideally placed to understand chemical reactions as a cause of fire, and she has carried out numerous post-fire contamination investigations throughout the world.